The Stem Cell Revolution
By Manisha Samy, ARK analyst
Discovered nearly thirty years ago in 1988[1], stem cell technology has failed to transform the field of regenerative medicine — until now. After delivering only one treatment during three decades of development, stem cell technology could finally become the all-purpose tool for repairing the body, thanks to rapid and precise genome editing techniques such as CRISPR and TALENs. Several obstacles in stem cell technology- high costs, safety concerns, and bioethical considerations – are beginning to fall away. In particular, the introduction of induced pluripotent stem cells (iPSCs) eliminated many of the initial bioethical concerns stirred by the the source of stem cells. Now, advances in genome-editing is accelerating the pace of progress.
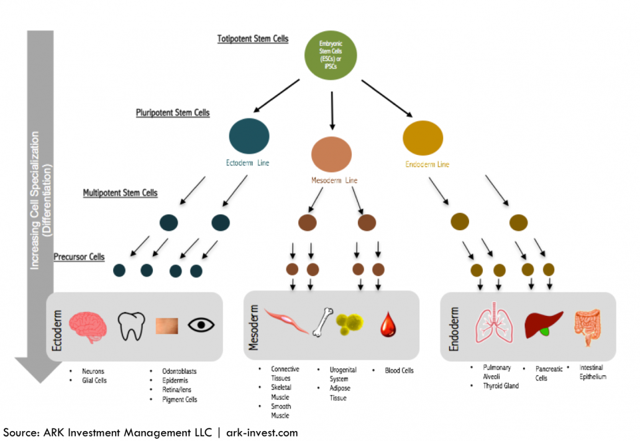
Stem cells exist in a “state of possibility”. Two key markers of stem cells are 1) the ability to self-renew and 2). The ability to become any specialized cell. Broadly speaking, a stem cell can evolve into any of the ~200 specialized human cell types, as illustrated in Figure 1. Directing stem cells to become any one of the 200 cell types has proven challenging.
Moreover, before the introduction of iPSCs, which are derived from adult cells, most human stem cells were sourced from human embryos or cord blood. The political and ethical controversies surrounding embryonic research curtailed stem cell funding. As a result, after a quarter century of research, bone marrow transplantation is the only FDA-approved stem cell application in the United States.
Figure 1
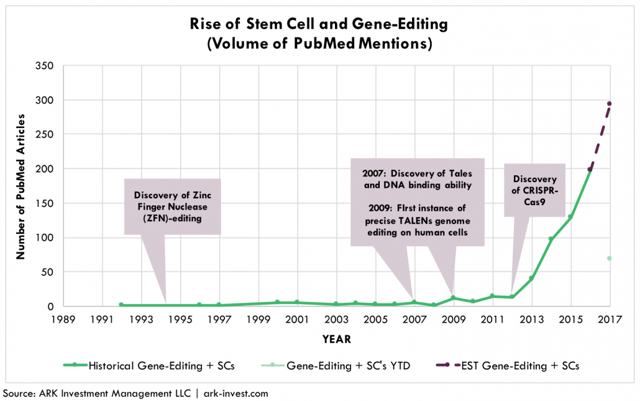
Breakthroughs in cheap, rapid genome-editing have re-invogored momentum in stem cell research. As shown in Figure 2, the number of publications on PubMed mentioning both stem cells and gene-editing hit a tipping point upon the discovery of CRISPR in 2012, three years after the first instance of precise TALENs-based genome-editing in human cells. Based on the current trajectory, ARK estimates that the number of scientific publications including both genome-editing and stem cells will approach 300 this year, accounting for nearly 20% of the total number of publications.
Figure 2
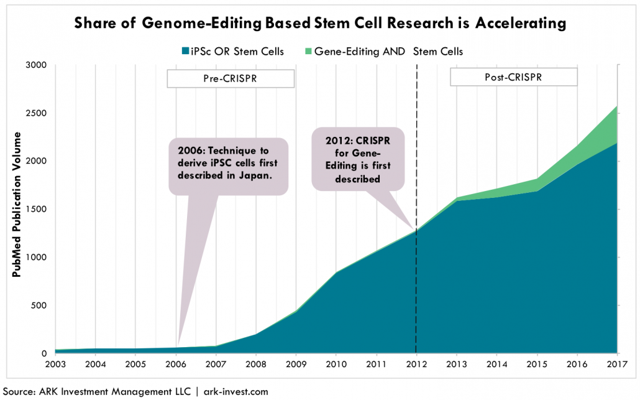
As illustrated in Figure 3, while the discovery of iPSC cells in 2006 boosted stem cell research noticeably beginning in 2008, the introduction of CRISPR in 2012 has further catapulted the stem cell field, with combined gene-editing and stem cell publications taking share.
Figure 3
Why Is The Addition of Gene-Editing So Important to Stem Cell Research and Therapy?
- Enhanced Safety Profile: CRISPR-edited stem cell therapies should offer a safer class of therapeutics than any other technology. While CRISPR technology is often criticized for off-target effects, they occur less frequently in iPSCs than in other cells lines. Moreover, with stem cell therapy alone, patients have to undergo chemotherapy, suffering dangerous side effects. CRISPR-edited stem cell therapy eliminates the need for chemotherapy, while mitigating off-target effects.
- Higher Efficiency and Lower Costs: iPSC cells spontaneously transform into more specialized cells, often making labwork quite difficult. With a shorter editing turnaround time, CRISPR lowers the probability that iPSCs differentiate into mature cells before the proper edits have been made, ensuring that the corrected genome is passed on to specialized cells. Typically, experiments require control tests, but replicating stem cell generated environments can be difficult. Alleviating that problem, CRISPR normalizes the genetic variability that exists across different iPSC cell lines. Additionally, CRISPRi, an application of CRISPR technology that silences genes instead of editing them, can reach efficiency levels of up to 95% in stem cells.
- Lower-Barriers to Entry: The number of scientific publications has increased because barriers to using genome-editing have decreased. A young research scientist faces a negligible learning curve and can be up and running in a week when choosing to use CRISPR technology. In fact, 51% of new CRISPR users are research scientists using the tool on stem cells for therapeutic purposes, as shown in Figure 4.
Figure 4
Ultimately, CRISPR edited iPSCs should unlock the code to human disease at a cellular level. Three public CRISPR companies are in a good position for the impending stem cell revolution. Editas Medicine (EDIT) signed a stem cell pact with GlaxoSmithKline (GSK) and Biogen (BIIB) for next-gen stem-cell therapies; CRISPR Therapeutics (CRSP)CRSP),+Casebia+Therapeutics+Sign+Commercial+License+Agreement+to+MaxCyte/12664463.html" rel="nofollow">licensed a CRISPR delivery mechanism for blood stem cells and has formed a CRISPR joint venture with Bayer (BAYZF); and Intellia Therapeutics (NTLA) has partnered with biotech giant Novartis NVS to focus on stem-cell based therapies. These companies, among others, finally might unleash the limitless possibilities that stem cells once promised in regenerative medicine, extending human life spans considerably.
Notes
[1] The Weissman Lab at Stanford University first isolated hematopoietic (blood) stem cells from mice bone marrow in 1988; it would be another decade before human stem cells were isolated.
Disclosure: ARK's statements are not an endorsement of any company or a recommendation to buy, sell or hold any security. For a list of all purchases and sales made by ARK for client accounts during the past year that could be considered by the SEC as recommendations, click here. It should not be assumed that recommendations made in the future will be profitable or will equal the performance of the securities in this list. For full disclosures, click here.
Disclaimer: ©2017, ARK Investment Management LLC ("ARK"). All content is original and has been researched and produced by ARK unless otherwise stated. No part of ARK's original content may be reproduced in any form, or referred to in any other publication, without the express written permission of ARK. The content is available for informational purposes only and is subject to change without notice. All statements made regarding companies or securities or other financial information on this site or any sites relating to ARK are strictly beliefs and points of view held by ARK or the third party making such statement and are not endorsements by ARK of any company or security or recommendations by ARK to buy, sell or hold any security. For a list of all purchases and sales made by ARK for client accounts during the past year that could be considered by the SEC as recommendations, click here. It should not be assumed that recommendations made in the future will be profitable or will equal the performance of the securities in this list. For full disclosures, please see the Terms of Use for this site.
READ FULL ARTICLE

Comments
Post a Comment